As of 2023, this major pharmaceutical had over 100k employees. The company is one of the world's largest biotech companies and focuses on a range of solutions for oncology, immunology, infectious diseases and opthamolgy.
The project run for this internationally renowned pharmaceutical company was investigating deviations due to a mixture of quality in the Filling Line. Deviations were addressed using an RCA methodology and were documented in a Quality Document Repository.
The knowledge and skill was within the team, but it did not always lie with the people who needed it, the result of this was that some RCAs were skillfully completed and documented, but the majority simply were not. It had been noticed that regulatory authorities that there were RCAs that did not meet the required standard.
We developed an approach which meant the lead investigators were typically drawn from a small group of compliance experts so concentrated effort with this group would make it possible to achieve a visible overall impact on the results.
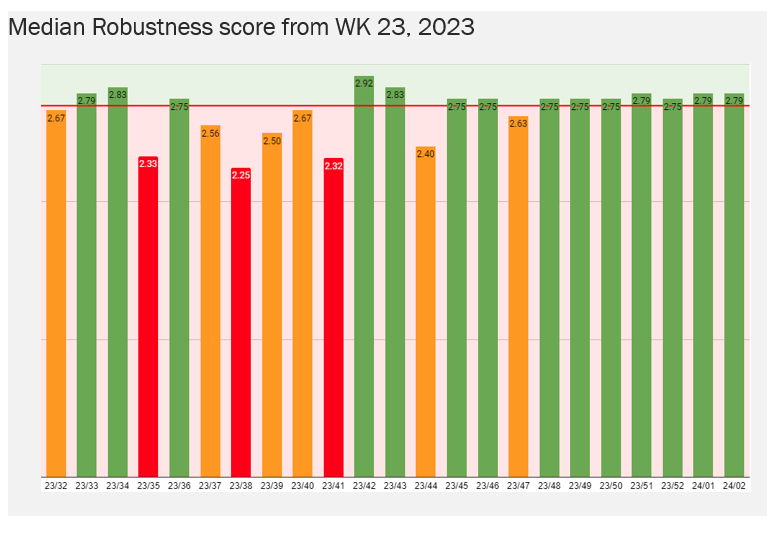
The impact was made visible by using a learning tool that evaluated each Deviation/RCA Robustness with a maximum score of 3.0 and acceptable score of >2.7.
Our approach was to: